Early Warning System for Cyanide Poisoning
Scientists from Jülich and Marburg develop biosensor for highly toxic cyanide
[6. April 2004]
Just five to ten bitter almonds can be lethal for a child. This is due to the cyanide compounds which are converted into highly toxic prussic acid in the human body. Cultivated plants such as apricots or beans also contain cyanide. Cyanides are used industrially for hardening steel and for corrosion protection. Just how dangerous this substance can be was demonstrated in 2000 in Romania. Cyanide solution used for extracting gold was released into the River Theiss and killed all living creatures along 300 kilometres of its course. There is now an "early warning system" for cyanide. Scientists from Jülich and Marburg have developed a biosensor that can detect cyanide swiftly and precisely well below the toxicity threshold. This biosensor could be applied at low cost in environmental and food monitoring. The scientists are now looking for an industrial partner to bring the existing prototype to commercial maturity.
Biosensors are measuring sensors that use a biological component - for example, enzymes or whole cells - to detect certain molecules or substances and to determine their quantities. They make use of the natural "lock-and-key" principle, according to which for the chemical conversion of a substance there is always an enzyme that "fits". In this way, the "cyanidase" enzyme degrades cyanide. The working group headed by Prof. Michael Schöning from Research Centre Jülich and Aachen University of Applied Sciences (Jülich Section) and Prof. Michael Keusgen's team from the University of Marburg connected this enzyme to a special semiconductor chip. For the cyanide, which was broken down by the cyanidase, they obtained a measurable electrical signal and thus a means of detecting even the smallest traces of cyanide.
"The semiconductor chips are in direct contact with the solution that is to be examined with respect to its cyanide content", explains Michael Schöning. "The cyanidase decomposes the cyanide into formic acid and ammonia, thus altering the pH of the solution. This change is recorded by the semiconductor chip as a capacitance drift. The "lock-and-key" principle of the cyanidase ensures that the recorded substances really originate from the cyanide and not from some other source."
In the joint project, the Jülich researchers are responsible for manufacturing the semiconductor chips with a special "EIS layer structure". EIS stands for electrolyte, insulator and silicon. The Marburg scientists are concerned with the cyanidase. "My group's contribution is the development, characterization and production of suitable cyanidase, as well as the development of methods for combining the enzyme with the EIS component," explains Michael Keusgen.
The intestinal bacterium Escherichia coli served the researchers as a host organism for the "production" of cyanidase. "This bacterium is used for producing quite different sorts of proteins. The best-known example is the production of human insulin. In biotechnology enzymes are produced by a very similar process ," says Michael Keusgen. However, the genetic information for the production of cyanidase originally stems from Pseudomonas bacteria that typically occur in the soil. Since they have to make do with all sorts of food sources, in the course of evolution these bacteria have developed the ability to use cyanide as an energy source.
For an adult human the consumption of about 50 milligrams of cyanide is lethal. The biosensor developed by Schöning and Keusgen responds to just one millionth of this quantity. Another advantage of the sensor is that it does not require any elaborate preparation of the sample to be examined. The two groups have already developed penicillin and garlic sensors on a similar principle.
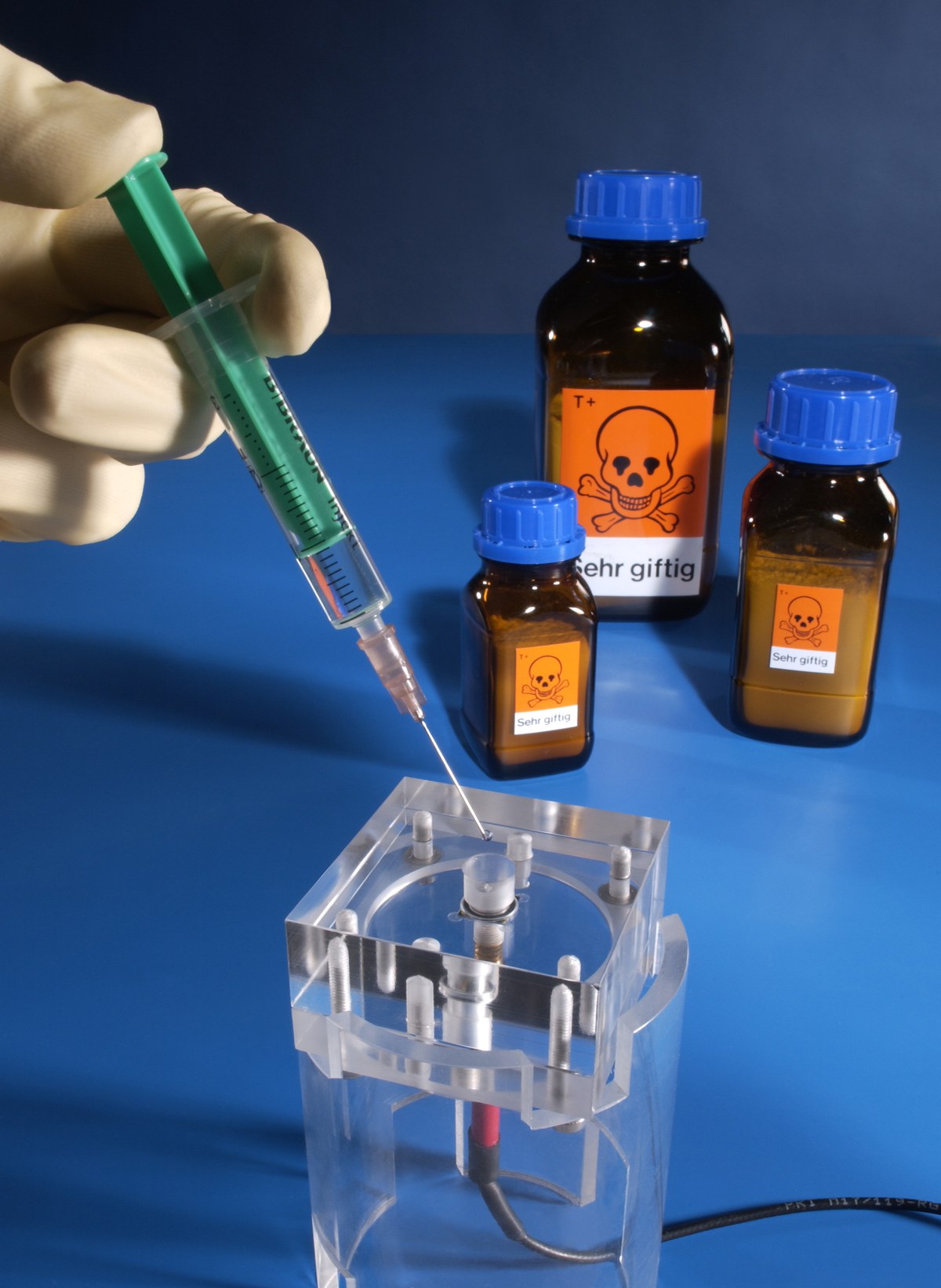
The solution to be examined with respect to its cyanide content is injected into the biosensor. Even minute quantities of highly toxic cyanide can be detected in this way.
Photograph: Research Centre Jülich
Further information:
Mechthild Hexamer
Head of the Public Relations Department and Press Relations Officer
Research Centre Jülich
52425 Jülich, Germany
Tel. ++49 2461 61-4661, Fax ++49 2461 61-4666
E-Mail: m.hexamer@fz-juelich.de
Dr Renée Dillinger
science journalist
Research Centre Jülich
52425 Jülich, Germany
Tel. ++ 49 2461 61-4771, fax ++49 2461 61-4666
E-mail:r.dillinger@fz-juelich.de
