Possible side effects of diesel bans
Nitrogen oxides are at the heart of the debate surrounding diesel vehicles – these are airborne pollutants that attack the mucous membranes and respiratory tract, and increase the risk of cardiovascular diseases. In order to comply with the thresholds set by the EU, cities could impose a ban on diesel cars. This would reduce the concentration of nitrogen oxides, particularly in busy areas. It is less well known that this may lead to an undesirable side effect – an increase in the formation of ozone, which also endangers health.
Far above in the stratosphere, at an altitude of 15 to 30 kilometres, ozone protects Earth from dangerous ultraviolet radiation. In the lower layers of the atmosphere it causes, for example, headaches, coughing, watery eyes and respiratory problems. These effects have long been known. Europe-wide limits apply to protect the population. “Just 20 years ago, there were regular ozone alerts in Germany,” says Franz Rohrer of the Forschungszentrum Jülich Institute of Energy and Climate Research (IEK-8). "Today, they are something of a rarity thanks to the air pollution control measures implemented in the late 1980s. Industry uses better filters and cars have become cleaner. Ultimately, this has resulted in lower ozone levels."
Ups and downs
Diesel driving bans could change all that. “One of the reasons for the decline in ozone pollution in busy areas is precisely the high levels of nitrogen oxides,” explains Rohrer’s colleague, Robert Wegener. Nitrogen oxides are one of the parameters in a complex series of chemical reactions that lead to the formation and subsequent decomposition of ozone. Two other factors are hydrocarbons and hydroxyl radicals (OH radicals). Hydrocarbons come from exhaust gases, but are also emitted by plants, while OH radicals are formed under the influence of sunlight.
Ozone is the product of a cascade of chemical processes that begins with the reaction of OH radicals and hydrocarbons. "Nitrogen oxides have an impact on this reaction," explains Wegener. "In low concentrations, they can accelerate the formation of ozone. However, if the quantity of nitrogen oxides is too high, these react directly with the OH radicals and the hydrocarbons play no part. This suppresses ozone formation."
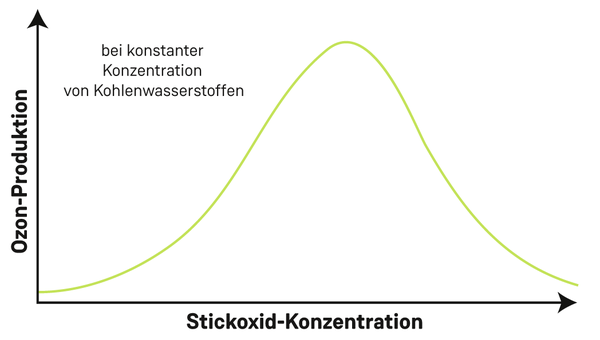
he key here is the ratio of nitrogen oxides to hydrocarbons. Put simply, if both substances are present in a certain ratio, a great amount of ozone will be produced as a result. “For a fixed amount of hydrocarbons, we can show this dependence using a simple graph. It looks like a mountain, with the peak denoting the ratio of nitrogen oxide to hydrocarbon that results in the greatest amount of ozone. To the left and right of the graph – that is with less or more nitrogen oxide – ozone production is reduced,” says Wegener.
A graphic similar to a topographic map can be obtained taking into account the concentrations of both nitrogen oxides and hydrocarbons. Here, the contours represent the rate of production of ozone at different concentrations of hydrocarbons and nitrogen oxides.
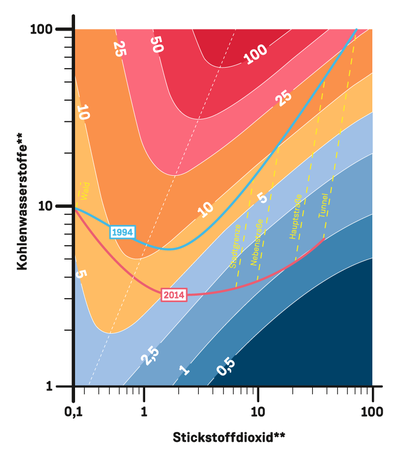
In the mid 1990s, the concentration of hydrocarbons and nitrogen oxides was particularly high. A large amount of ozone was being produced, to the point that the rate of production was in the red zone. With the introduction of catalytic converters, the hydrocarbon concentration dropped to one-fifteenth of its original level, while nitrogen oxide levels were only halved. The ratio of nitrogen oxides to hydrocarbons increased, and OH radicals were increasingly removed from the atmosphere by the nitrogen oxides, leading to the formation of less ozone. Today, we are in the blue zone on our ‘map’, indicating that the rate of ozone production is far lower than it once was.
But what will happen if future driving bans only reduce the nitrogen oxide emissions? “We will start moving to the left of the graph, and thus back up the mountain, resulting in more ozone being generated in areas with heavy traffic,” says Rohrer.
Andere Verhältnisse schaffen
Wie lässt sich das verhindern? Man müsste geeignete technische Maßnahmen bei der Abgasnachbereitung entwickeln. So könnte man – zusätzlich zu den reduzierten Stickoxiden – die Menge der Kohlenwasserstoffe weiter verringern. "Das könnte erreicht werden, indem man zum Beispiel das Kaltstartverhalten von Autokatalysatoren weiter verbessert", so Rohrer. Alternativ können bauliche Veränderungen lokal hohe Konzentration von sämtlichen Luftschadstoffen senken, weiß Wegener: "Die Stickoxide sind sehr kurzlebig – und damit nur am Ort ihrer Entstehung gesundheitsgefährdend. In den Häuserschluchten der Großstädte halten sie sich nur für ein paar Minuten, bevor sie sich weiterverteilen. Wenn die Luft schneller durch die Straße strömen würde, wären sie kein Problem". Manche Städte gehen diesen Weg. So werden zum Beispiel einzelne Häuser in Reihenhaussiedlungen entfernt. Dadurch entsteht ein Querwind; die Stickoxide – und andere Schadstoffe – verteilen sich. In modernen asiatischen Städten etwa werden heute solche Effekte schon von vornherein beim Bau eingeplant. Auch dadurch entsteht weniger Ozon.
Complex processes
But the ratio of nitrogen oxides to hydrocarbons is not the only parameter affecting the formation of ozone. This is a highly complex process, due to the fact that it involves thousands of different hydrocarbons – all with different lifetimes and chemical structures. Some have a strong effect, while others are not involved in the formation of ozone at all. The same applies to nitrogen oxides. The most significant of these are nitric oxide and nitrogen dioxide, which each affect the formation of ozone in different ways.
In addition, external conditions such as climate zone, wind speed and direction, solar radiation and day- or night-time also play an important role.
The need for reliable data
"Specific regional conditions must be taken into account to determine how the air quality can be improved in a particular location. For this, differentiated measurement data and reliable computer models are needed," says Rohrer. The climate researchers at Forschungszentrum Jülich use their MobiLab, a measuring vehicle, to acquire data about the current pollution levels in German cities. Exactly how are nitrogen oxides and other pollutants distributed within the major cities? Where are the most polluted areas? What are the causes? By finding answers to these and similar questions, they hope to one day be able to reliably determine whether a driving ban makes sense in a given place.
Regine Panknin
Ozone production over the past years with regional variations:
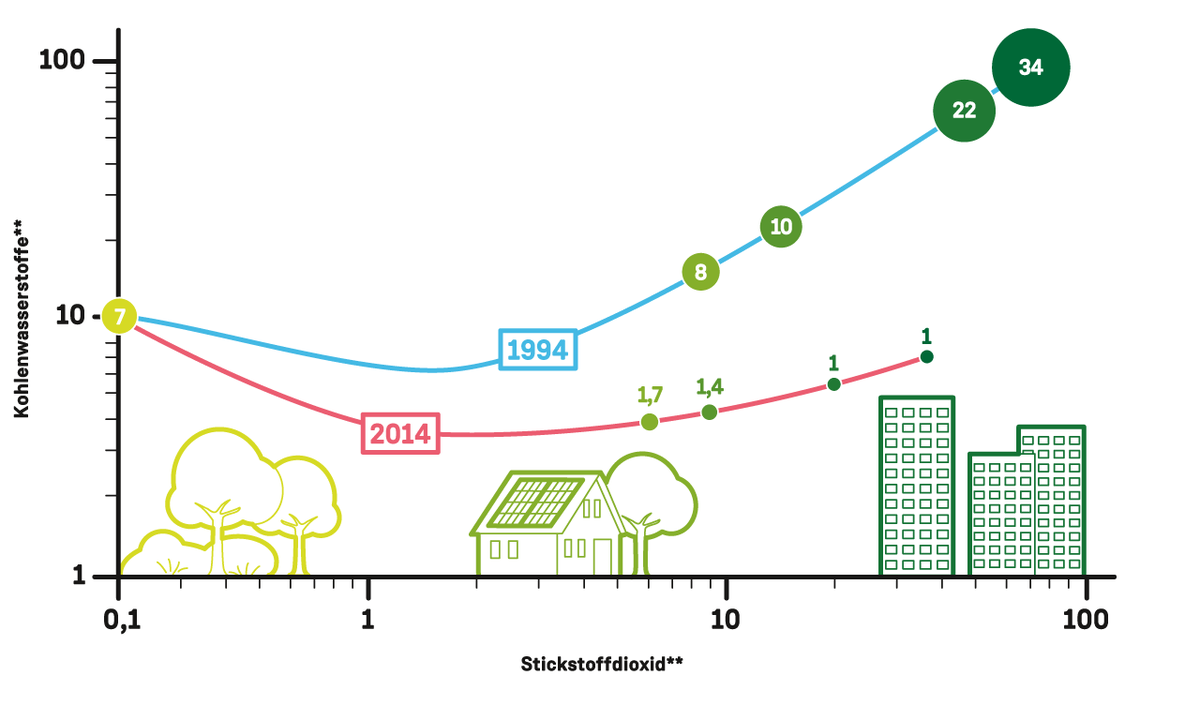
Near-ground ozone production (green circles, ppb/h - parts per billion per hour) depends on the ratio of nitrogen dioxide concentration (bottom axis) to hydrocarbon concentration (left axis) – see diagram above.
Over the past years, Germany’s overall ozone pollution has decreased, but with significant regional variations (comparative data for 1994 and 2014). While in the suburbs (green) and inner city areas (dark green) the very high levels dropped considerably, they have remained almost unchanged in rural areas (light green).
Inner cities
The extreme decline in inner cities is due to the introduction of catalytic converters for motor vehicles. As a result, the hydrocarbon concentration fell to one fifteenth of its previous level, while the nitrogen oxide levels were halved. The altered ratio of these substances means that much less ozone is now being generated in busy areas.
Suburban areas
The air composition is different in rural regions and suburban areas. There, the hydrocarbons in the air come largely from vegetation and less from vehicle exhaust gases. The concentration has fallen less here, so ozone production has not decreased to such a degree.
Der extreme Rückgang in den Innenstädten liegt an der Einführung von Katalysatoren für Kraftfahrzeuge. Dadurch sanken die Kohlenwasserstoff-Konzentrationen auf ein Fünfzehntel, die Stickoxidwerte auf die Hälfte. Das veränderte Verhältnis der beiden Stoffe führte dazu, dass heute in verkehrsreichen Gebieten viel weniger Ozon erzeugt wird.
Ländlicher Raum
Im ländlichen und stadtnahen Raum ist die Luftzusammensetzung anders. Dort stammen die Kohlenwasserstoffe in der Luft zu großen Teilen aus der Vegetation und weniger aus Abgasen von Fahrzeugen: Die Konzentration ist weniger gesunken und die Ozonproduktion deshalb nicht so stark zurückgegangen.
